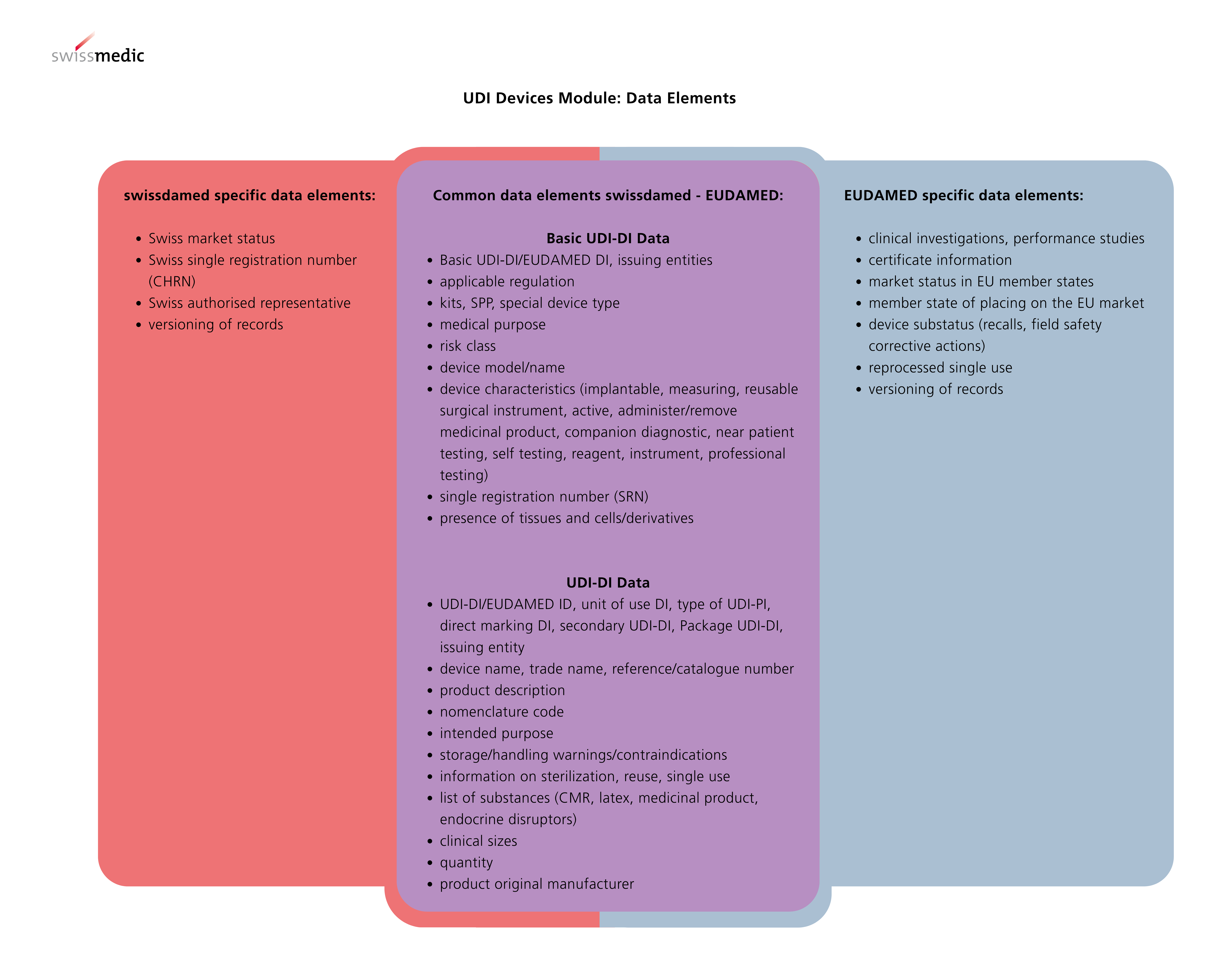
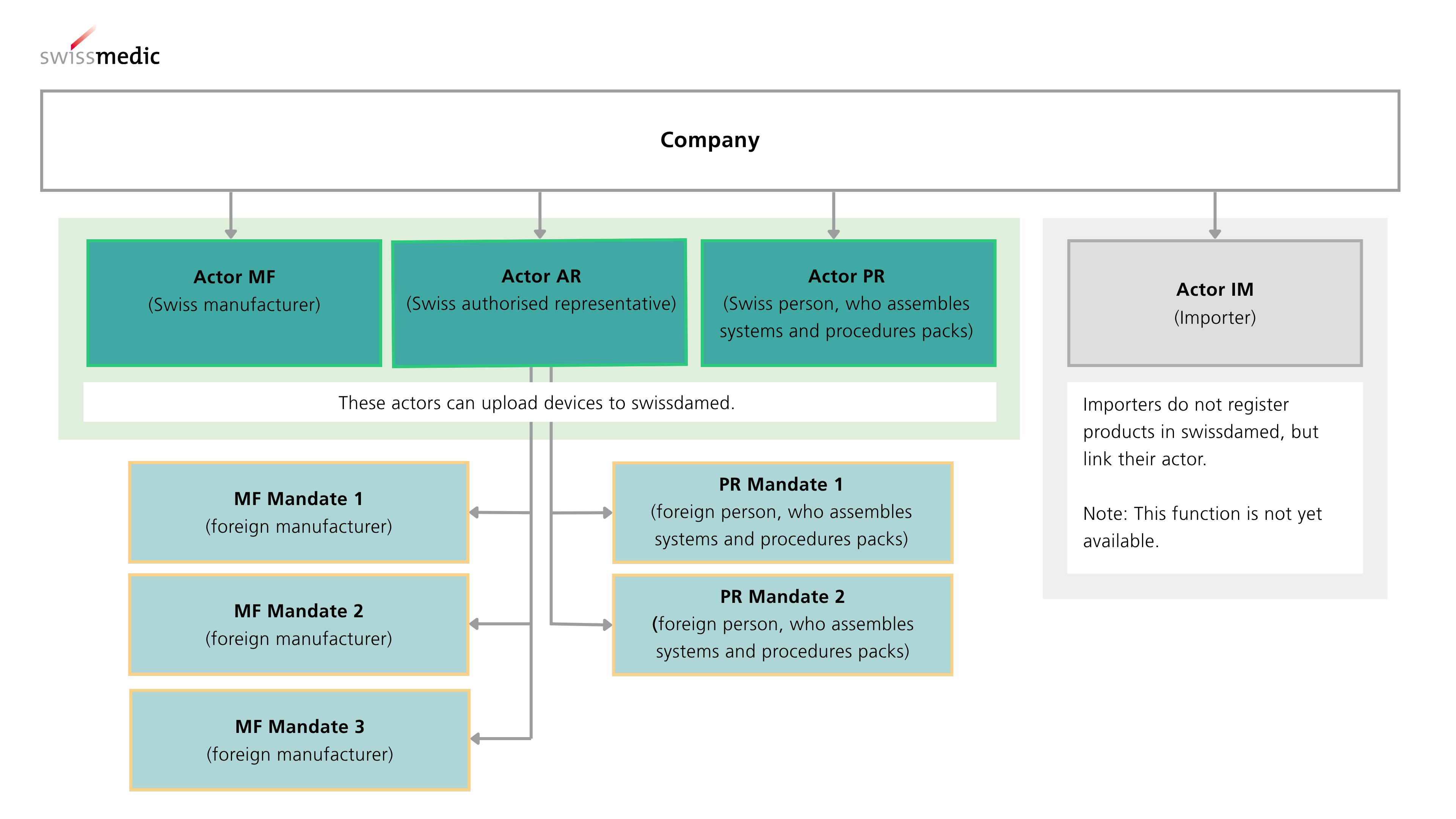
As of 1 July 2026, all devices, systems and procedure packs that are placed on the market in Switzerland from this date must be registered in the swissdamed UDI Devices module.
The registration obligation applies to devices under the existing legislation (MedDO, IvDO) and to devices under the old legislation according to Art. 101 MedDO and Art. 82 IvDO (“legacy devices”), which continue to be placed on the market at the time the registration obligation enters into force.
Devices, systems or procedure packs that are placed on the market after the entry into force of the registration obligation on 1 July 2026:
- must be registered before they are placed on the market
- however, a transitional period applies until 31 December 2026*
Devices, systems and procedure packs that were placed on the market before the entry into force of the registration obligation on 1 July 2026 and continue to be placed on the market after the transitional period (31 December 2026):
- must be registered in swissdamed at the latest by the end of the transitional period for the registration obligation (31 December 2026)*
The registration process shall be in accordance with the provisions of Art. 17 para. 5 MedDO and Art. 16 para. 5 IvDO (AS 2024 742 - Ordinance on In Vitro Diagnostic | Fedlex), which enter into force on 1 July 2026.
* Exception: Immediate registration without a transitional period will apply from 1 July 2026 for devices and systems and procedure packs for which a serious incident, field safety corrective action or trend must be reported to Swissmedic.
In general, all device data registered in swissdamed can be viewed by the public.