swissdamed – the Swiss Database on Medical Devices – is a Swissmedic database for registering economic operators and medical devices, including in vitro diagnostic medical devices, on the Swiss market*.
swissdamed aims to provide an overview of the medical devices, including in vitro diagnostic medical devices, available on the Swiss market and the economic operators responsible for them. The database gathers and publishes information about medical devices and the companies concerned (manufacturers, authorised representatives, importers).
swissdamed provides a publicly accessible website with a search function. It has been designed to give healthcare professionals and the public access to information about the medical devices and in vitro diagnostic medical devices placed on the market in Switzerland and about the economic operators responsible for them, and thereby increase transparency.
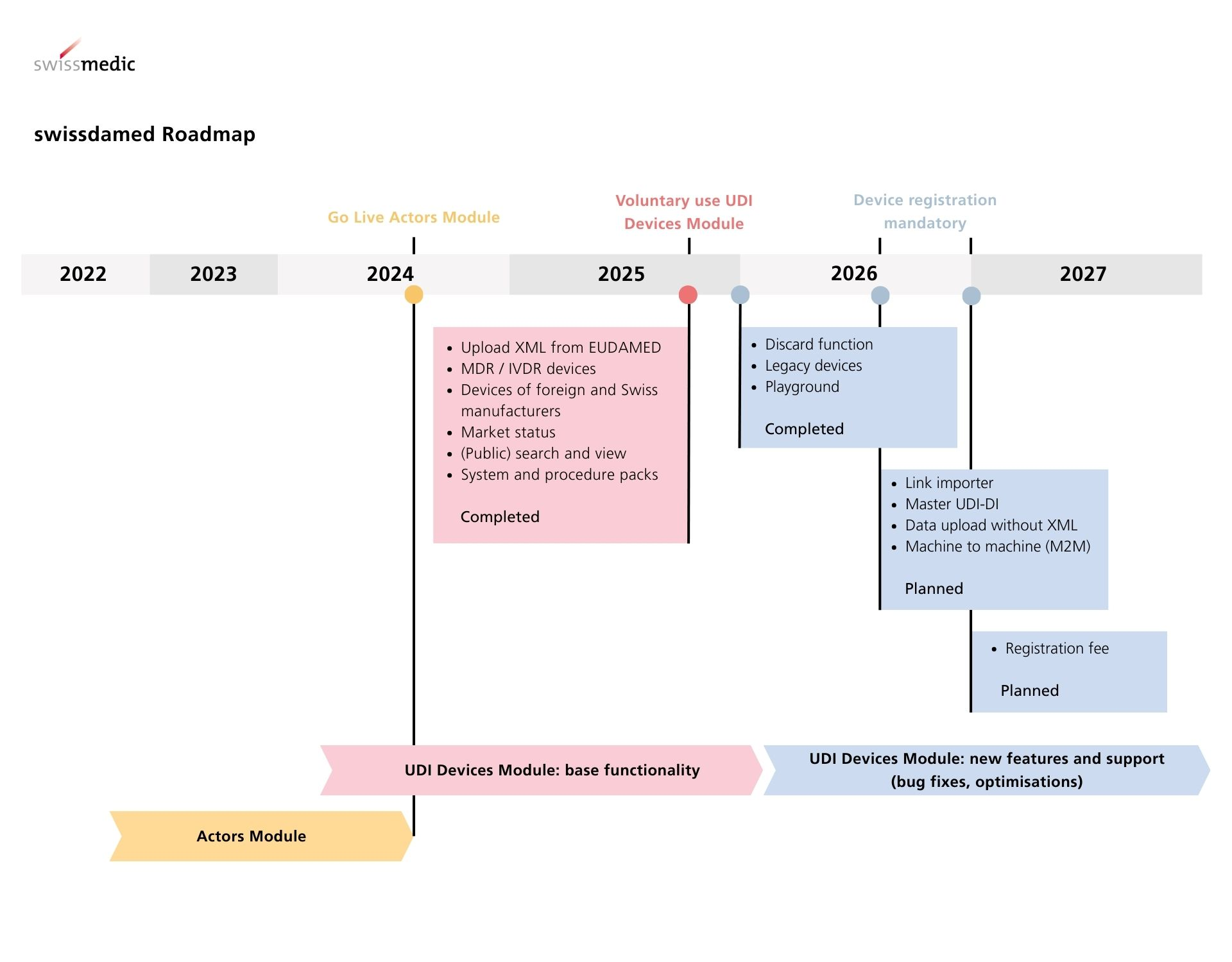
swissdamed consists of two modules, the "Actors module" and the "UDI Devices module”. In order to preserve maximum equivalence between the Swiss and EU regulations and minimise the effort for economic operators, swissdamed has aligned its modules to the corresponding modules of the European database EUDAMED.
Since there is no interface between swissdamed and EUDAMED, Swissmedic cannot import or synchronise data from EUDAMED. Swiss manufacturers, persons who assemble systems and procedure packs, and authorised representatives must actively upload device data to swissdamed. Currently, an XML file in EUDAMED 'GET DEVICE' or 'POST DEVICE' format can be used for this purpose. Further upload methods, for example machine-to-machine, will be possible at a later date.