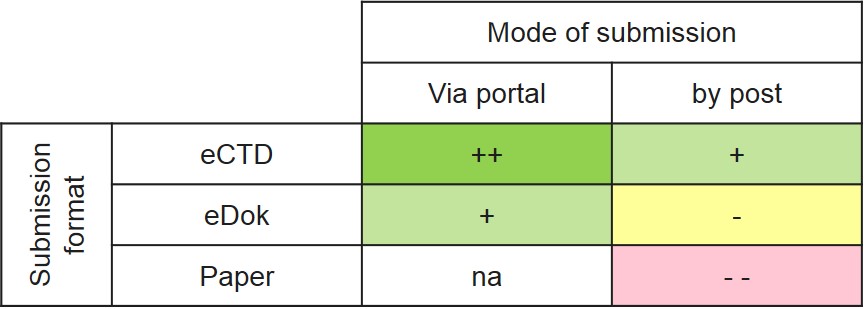
Submissions for authorisation applications can be submitted to Swissmedic in the eCTD and eDok formats and on paper, either via the eGov portal or by post.
For reasons of efficient processing and the quality of the submitted documentation, Swissmedic prefers submissions via the portal in the eCTD format.