In scientific matters, Swissmedic obtains specialist advice from the Swissmedic Medicines Expert Committees (SMEC). These consist of the Human Medicines Expert Committee (HMEC) in the case of medicinal products for human use and the Veterinary Medicines Expert Committee (VMEC) where veterinary medicinal products are involved. The members of both bodies are appointed by the Agency Council of Swissmedic.
The SMEC support Swissmedic by providing expert reports and advice on the scientific assessment of documentation relating to the authorisation, market surveillance and approval of medicinal products. They perform these activities by answering specialised questions posed both in relation to and independently of pending cases.
To ensure impartial advice, each member of the Swissmedic Medicines Expert Committees discloses his or her interests on an annual basis. In addition, before a review or advisory mandate is awarded, it is necessary to check whether any conflicts of interest exist in light of the vested interests that have been disclosed. The Code of Conduct of the Members of the Swissmedic Medicines Expert Committees defines the treatment of vested interests and how to proceed in the event of any conflicts of interest.
Contact
Human Medicines Expert Committee (HMEC)
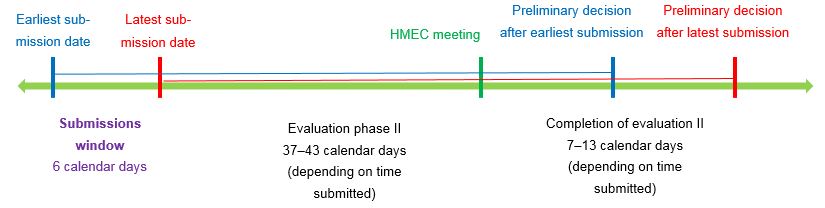
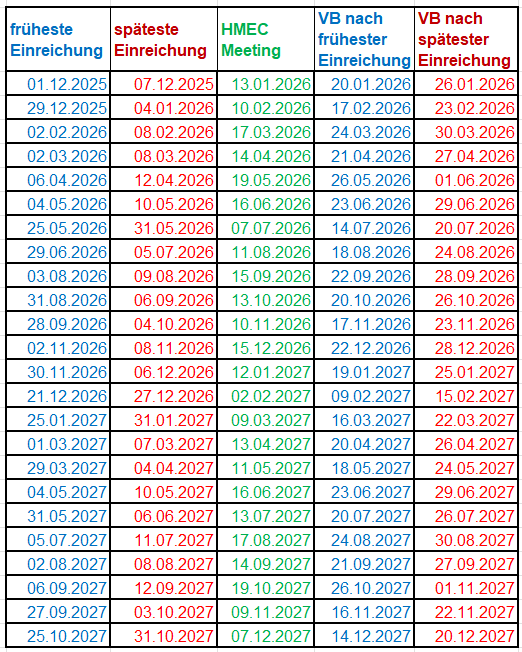
Publication of time windows for submitting replies to the List of Questions for applications in the fast-track and temporary authorisation procedures
To ensure that applications are processed efficiently and promptly, we are supplying the dates of HMEC meetings below.
For all applications for the fast-track authorisation procedure as well as new applications for temporary authorisation, the submissions window must be observed when transmitting responses to the List of Questions.
Members/Public Declaration of Interests
Documents

