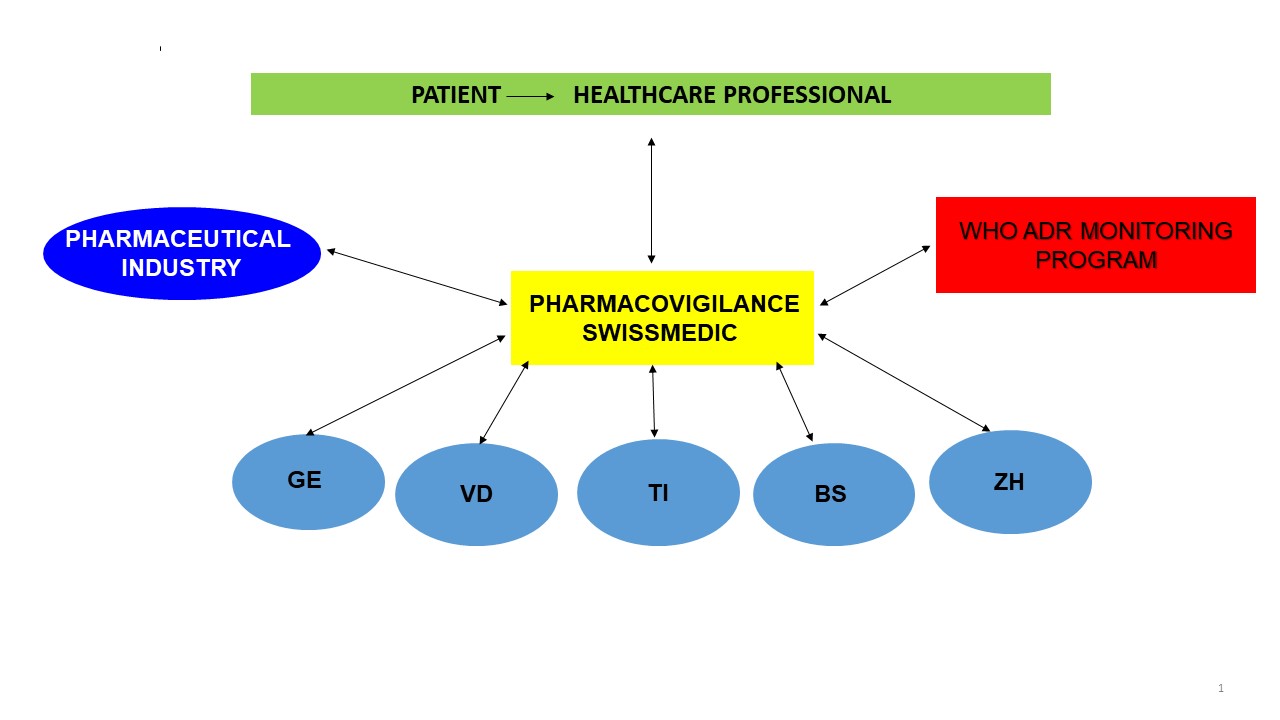
Need for pharmacovigilance
Medicines are developed over a period of several years. The efficacy and safety of a new drug are generally studied on a few thousand carefully selected and followed-up trial subjects and patients according to strictly defined criteria. For this reason only very frequent adverse reactions – mainly those depending on the drug's pharmacological properties – can be observed during its clinical development.
Once the product has been placed on the market, a much larger – and also often polymorbid – population will be exposed, which may lead to a change in the drug's hitherto known safety profile. Adverse drug reactions can then be observed more frequently, including those occurring only sporadically and independently of the pharmacological properties of the substance. These adverse reactions of drug treatment observed in daily practice must be reported without delay. If such information is consistently forwarded to the Swissmedic National Pharmacovigilance Centre, hitherto unknown risks can be identified and tackled.