18.01.2019
Swiss M1 Specification and Swiss eCTD Validation Criteria
As announced on 12.12.2018
the corrected eCTD specification documents are now being made available.
The following corrections have been made:
Swiss M1 Specification
The notation of the new elements in Section 1.12, Table 4 is incorrect and have been brought into line with the correct notation in the DTD:
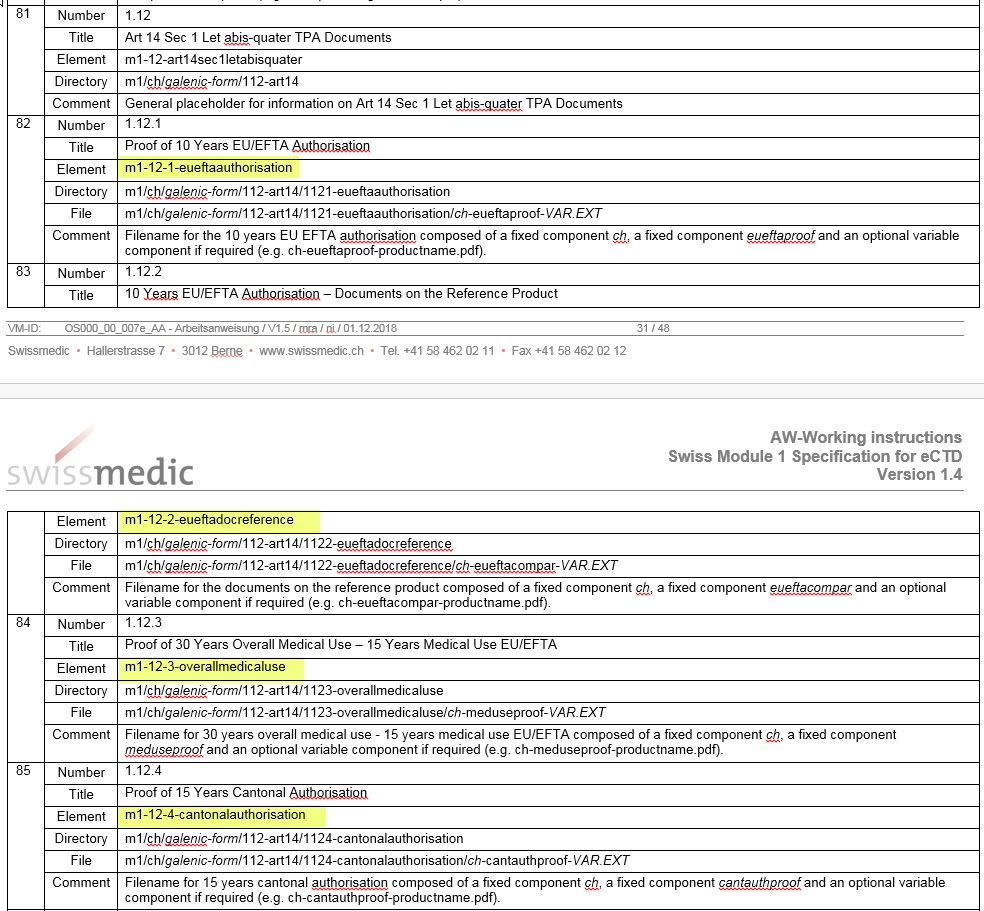
- 1.8.2 Risk management system. The hyphen was missing in Table 1 and has been reinserted

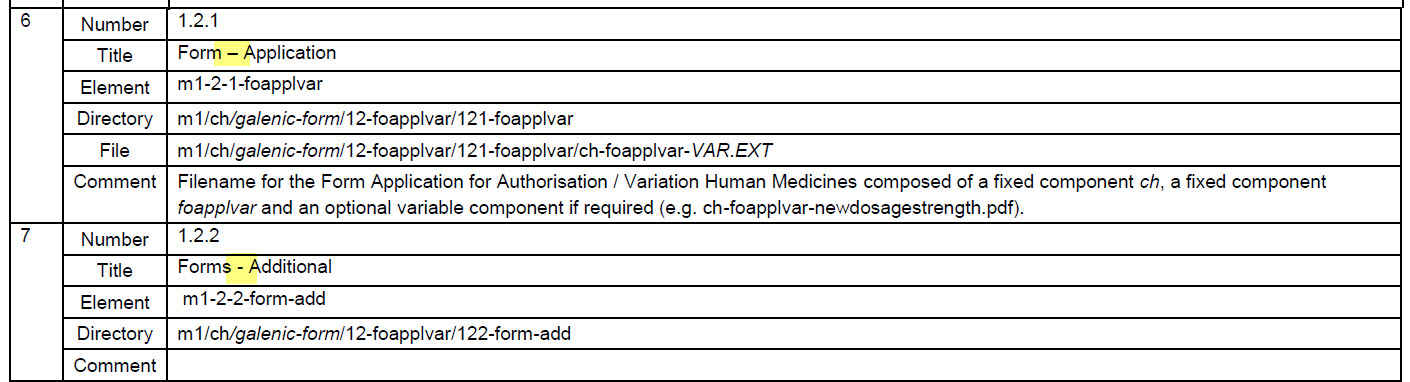
- The hyphen in 1.2.1 is different to that in 1.2.2 in Table 4. The notation of the hyphen has been adjusted
- Swiss eCTD Validation Criteria
- The folder name 151-infoaccordappIVguidelinebioequivalence is incorrect and should be 151-infoaccordappivguidelinebioequivalence. IV was wrongly capitalised.

Please note that due to technical reasons, the version number 1.4 will be retained.
The Questions & Answers Swissmedic eCTD Implementation information sheet is also being updated. It includes information and clarification on the use of eCTD in relation to the changes in the M1 Specification.

