27.02.2019
Update: Correction of the new eCTD specification documents Swiss M1 Specification and Swiss eCTD Validation Criteria
Due to minor errors in the eCTD M1 Specification and the corresponding eCTD Validation Criteria, both of these documents require correction. For technical reasons, the version number 1.4 will be retained.
In addition, we are providing more detailed explanations here to clarify a number of points.
The following corrections were made to Version 1.4:
- In the document eCTD Validation Criteria:
- The folder name 151-infoaccordappIVguidelinebioequivalence is incorrect and should be 151-infoaccordappivguidelinebioequivalence. IV was wrongly capitalised

- In the document Swiss M1 Specification:
- The notation of the new elements in Section 1.12, Table 4 is incorrect and will be brought into line with the correct notation in the DTD:
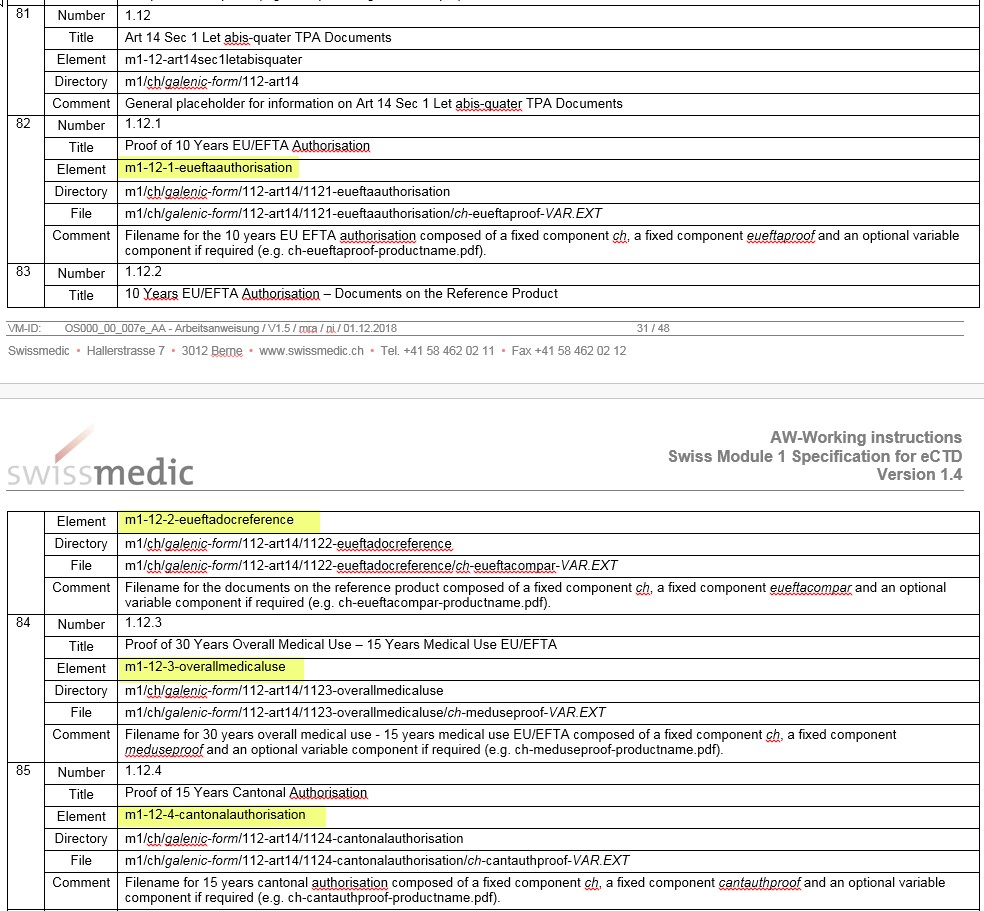
- 1.8.2 Risk management system. The hyphen was missing in Table 1 and has been reinserted

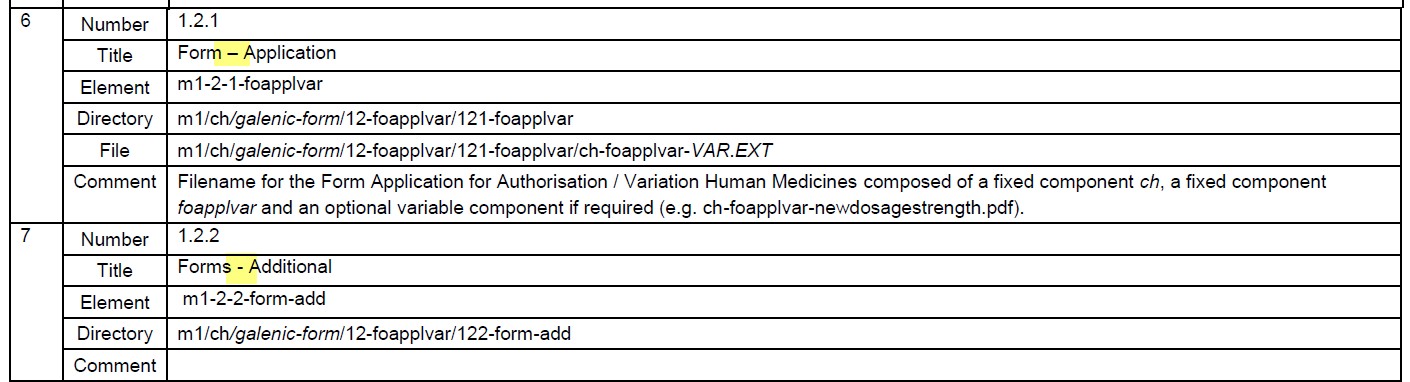
- The hyphen in 1.2.1 is different to that in 1.2.2 in Table 4. The notation of the hyphen has been adjusted
The following corrections have been noted for the future version 1.5 and will be subsequently corrected in that version. The publication date of version 1.5 is yet to be announced.
- In the document Swiss M1 Specification:
- In Table 1 under 1.2.2, no fixed component of filename and no life cycle operator are necessary

- In line with HMV4, application types pi and eas are no longer necessary and will be deleted from the table.
- In the document eCTD Validation Criteria:
- Incorrect entry in the file folder structure and names. There are differences between the M1 Specification and the Validation Criteria. The fixed component of 1.2.2.20 should be foart13.

In response to questions addressed to Swissmedic, our answers are as follows:
How should a Life Cycle take place in connection with deleted sections (e.g. 1.2.2.4, 1.2.2.5 etc.)? Deleted sections are marked with "this section is no longer applicable..." in the M1 Specification.
Are the following notations in document Swiss M1 Spec, Table 1 and Table 4 correct?
Answer:
- Operation replace/delete in an existing document in a deleted section -> If there is a Life Cycle, the rule 15.BP3 takes effect and issues a warning. The sequence remains valid.
- Operation new in a deleted section -> no Life Cycle should take place any longer. The rule 15.BP3 issues a warning.
- Generally speaking, documents that were previously allocated to these deleted sections are either no longer up to date or have been replaced by new forms in another section.
- Life Cycle in an existing document in the recently deleted section 1.5.3 -> a Life Cycle is not anticipated here.
Answer:
- 1.2.2.16 PSUR/PBRER form for Human Medicines = notation without spaces is deliberate
- 1.2.2.23 Application for Recognition of Orphan Drug Status form = capitalisation of Recognition is deliberate
- 1.7.4 FDA Decision = use of singular is deliberate
- 1.4.1 Quality (Leaf) = omission of details regarding Expert is deliberate
- 1.4.2 Nonclinical (Leaf) = omission of details regarding Expert is deliberate
- 1.4.3 Clinical (Leaf) = omission of details regarding Expert is deliberate

