Swissmedic has been the focus of attention since the COVID-19 pandemic emerged. It is expected to take rapid decisions about vaccines and therapeutic agents and to perform post-marketing surveillance of medicinal products. On 19 December 2020, Swissmedic became the first western authority to authorise a COVID-19 vaccine in a normal procedure.
20.12.2021
To be granted authorisation for a medicinal product, the applicant must submit data from scientific studies. The manufacturer also requires a licence to produce the medicinal product in Switzerland (the Spikevax vaccine from Moderna, for example, is produced by Lonza AG in Visp). The submitted data are reviewed by Swissmedic using internationally recognised criteria in respect of quality, safety and efficacy.
If the outcome of this review is positive and the benefit outweighs the risks, the medicinal product is authorised and can be brought onto the market. After a medicinal product has been authorised, Swissmedic and the authorisation holder must continue to monitor its safety, efficacy and quality. Swissmedic does this by evaluating national and international reports of side effects and, where necessary, taking action to minimise risks for the population.
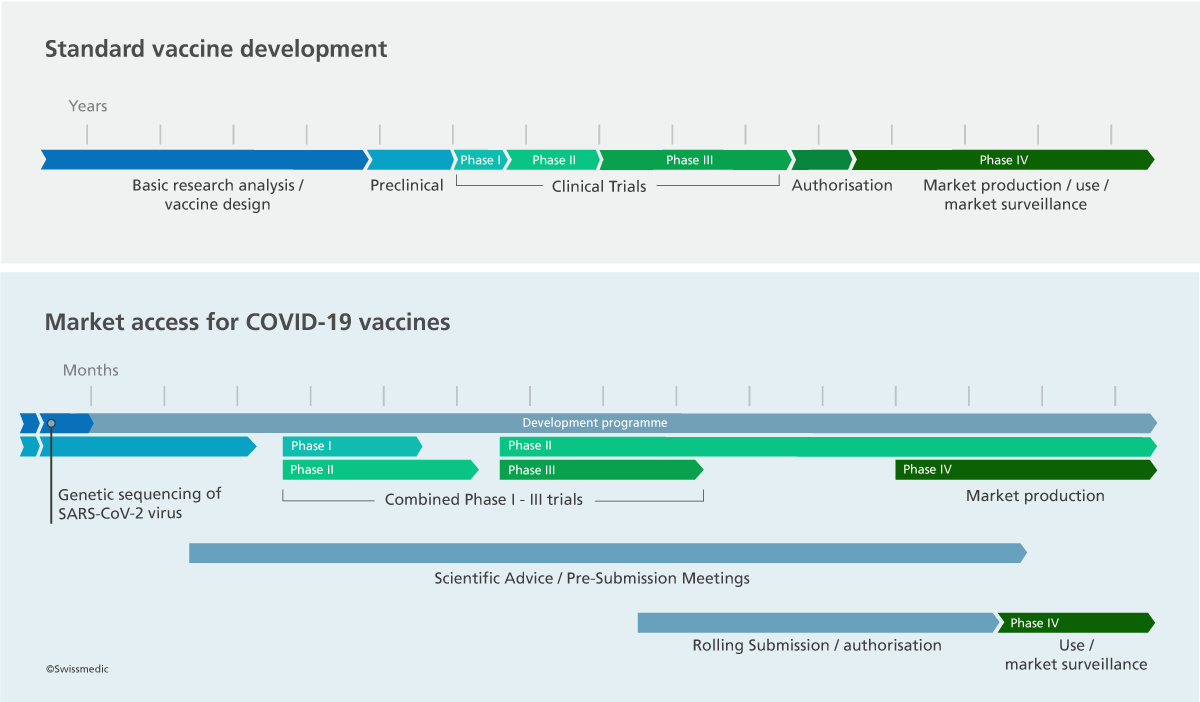
The authorisation timeline
The timeline for authorisation is determined to a very large extent by the applicant. The decisive factor in efficient evaluation and in reaching a decision on authorisation is the quality of the scientific data. This includes rapid responses to questions sent to the company by Swissmedic. Another important element is the time at which the authorisation application is submitted. Switzerland has a small sales market and is therefore rarely the country of first choice.
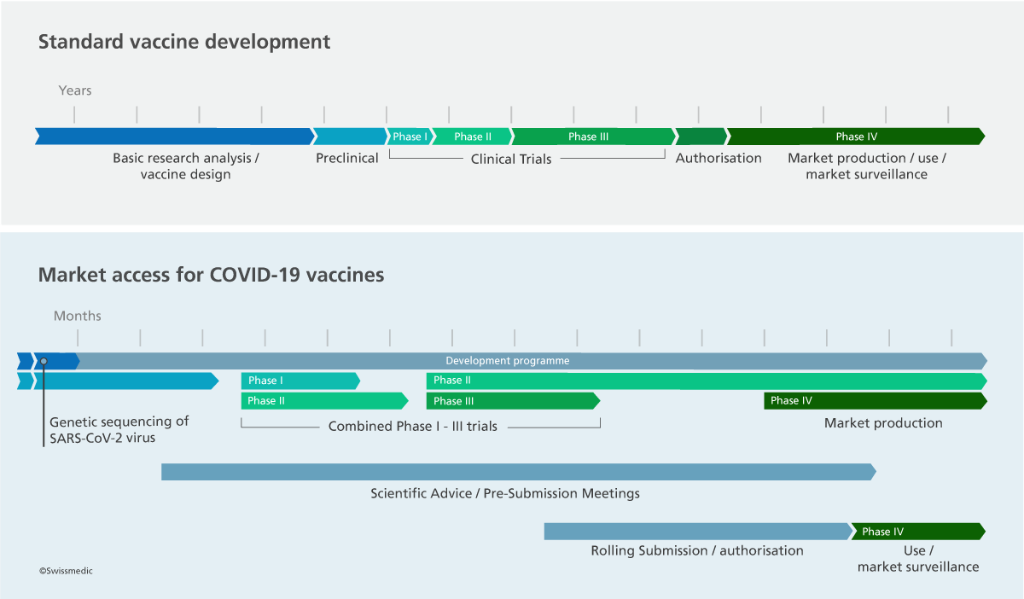
How to gain time: a rolling submission speeds up processes
The option of a rolling submission was introduced for COVID-19 medicinal products in view of the extraordinary pandemic situation. This is a special form of the authorisation procedure designed to ensure the rapid authorisation of urgently needed medicines. Applicants are not required to include a complete dossier with their initial submission to Swissmedic. Instead, they submit the first available data packages along with a schedule for submitting subsequent data packages.
The advantage of a rolling submission is that Swissmedic can start evaluating the initial data packages at an early stage and can clear up outstanding issues and questions with the applicant on an ongoing basis. This enables Swissmedic to gain a comprehensive picture of the benefit-risk profile of a medicinal product before the last clinical trials have been completed.
Swissmedic can take a rapid decision on authorisation as soon as all the available scientific evidence has adequately demonstrated the efficacy, safety and quality of the product and the benefit has been shown to outweigh the risks.
If the clinical trials have not been completed by the time the product is authorised, Swissmedic issues an ex officio temporary authorisation. This obligates the company to submit the final results of the clinical trials within two years. If these results support the safe and effective use of the product, the temporary authorisation can be transformed into an ordinary authorisation.
Applicants may also contact Swissmedic before they submit an application. The Scientific Advice and Pre-Submission Meetings offered free of charge by Swissmedic during the pandemic provide companies with the best possible advice and support in all scientific, regulatory and organisational matters.
Authorisation applications for COVID-19 vaccines were also handled on a rolling basis in other countries. The efficiency of this approach is illustrated by the fact that, on 19 December 2020, Swissmedic became the first western authority to authorise a COVID-19 vaccine in a normal procedure.
Collaboration with partner authorities
The Therapeutic Products Act enables decisions issued by foreign regulatory authorities to be taken into account. In this case, Swissmedic still takes an independent decision while basing it on the assessment reports from partner authorities. This enables Swissmedic to ensure that the safety of patients in Switzerland is not compromised. Responsibility and liability issues cannot be delegated to another country or another therapeutic products agency.
As a contributor to economic and safety surveillance, Swissmedic continues to monitor the market after products have been authorised. Moreover, by implementing regulations, performing inspections and compiling reports it ensures that the therapeutic products market is safe.
Different types of authorisation
Swissmedic reaches its decisions on the basis of the legal requirements in the normal procedure. This means that only the scientific data that have been submitted may be analysed and taken into consideration in reaching a decision on authorisation.
Other countries (such as the USA and the United Kingdom) employ something known as emergency authorisations. These enable the overall pandemic situation in a country to be taken into account in addition to the submitted data. The legislation in Switzerland explicitly does not foresee this possibility: the intention is to ensure the greatest possible degree of independence for the therapeutic products agency Swissmedic.