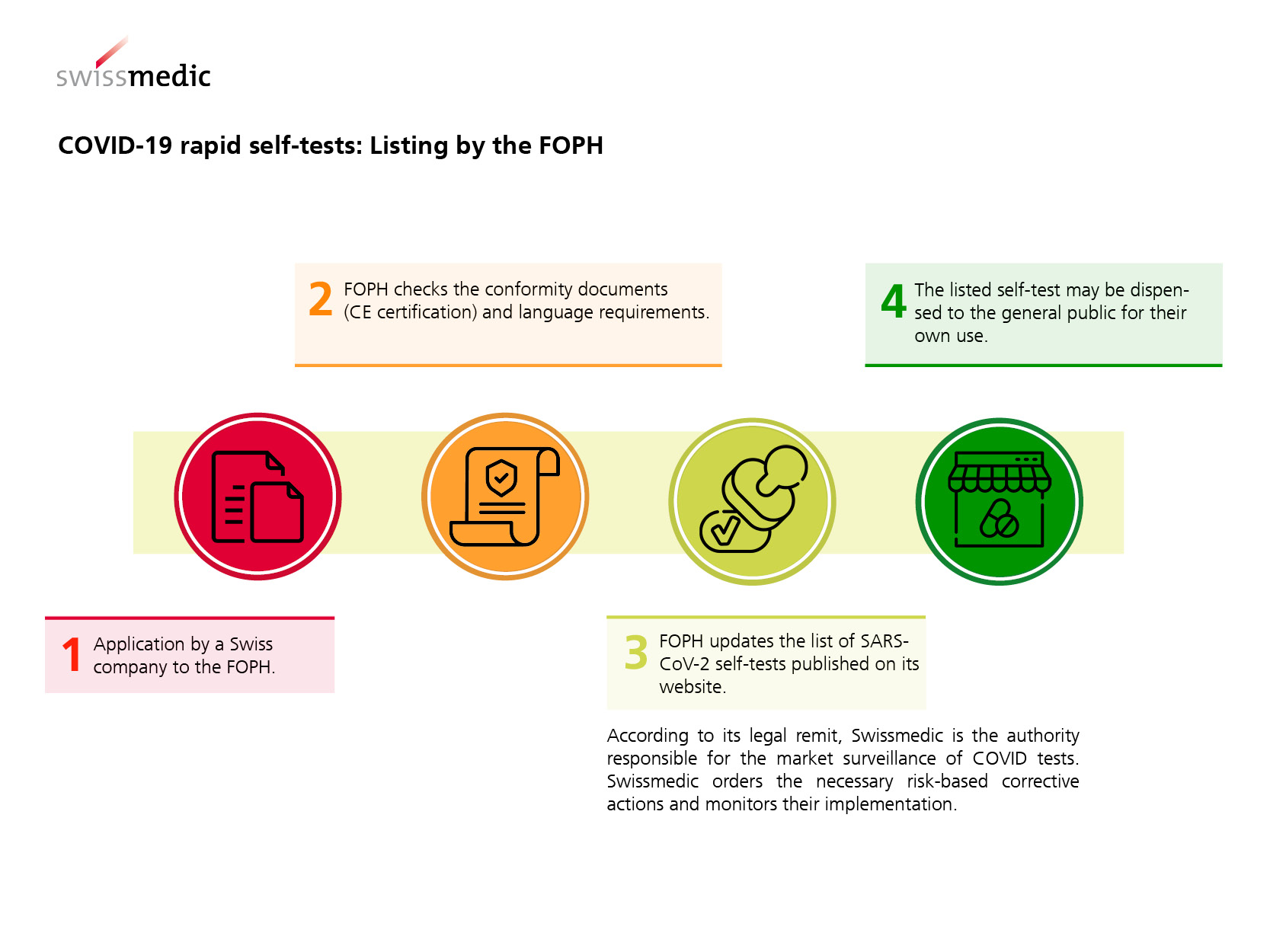
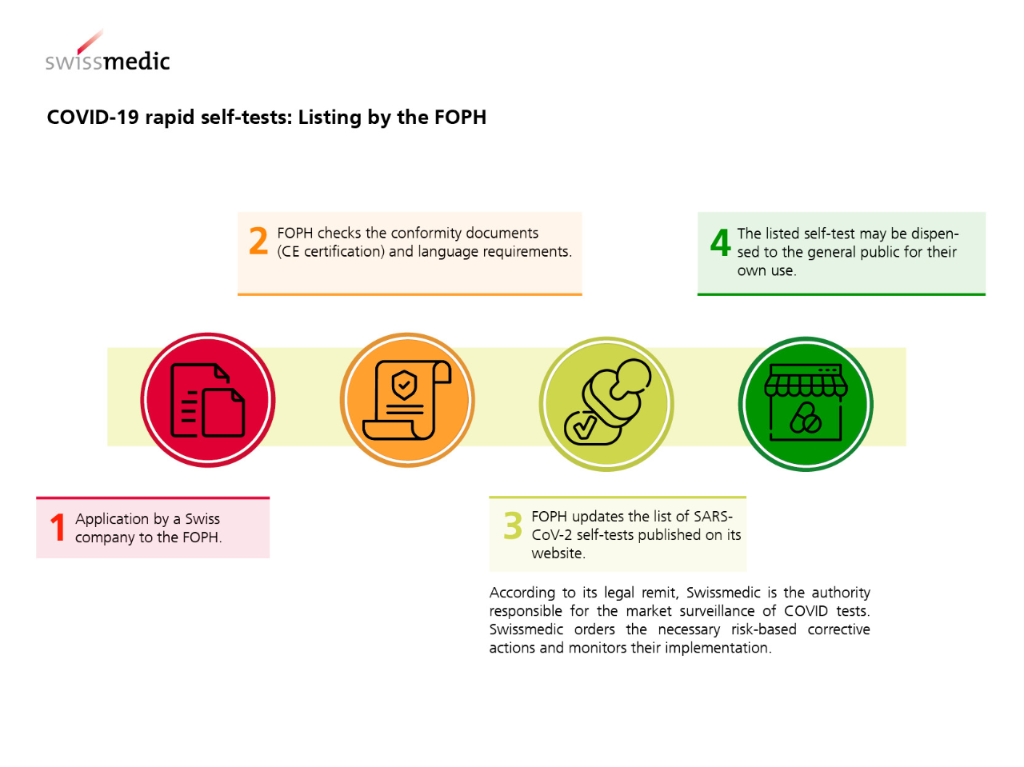
In Switzerland, COVID-19 rapid self-tests for private individuals have been available in pharmacies since 7 April 2021 and through other distribution channels since 26 June 2021. Only tests* that carry a CE marking and have been certified for self-use by a designated/notified body may be dispensed. On its website, the FOPH publishes a list of the rapid tests for self-use that are authorised in accordance with COVID-19 Ordinance 3.
COVID-19 tests are classified as in-vitro diagnostic medical devices for the detection of transmissible diseases. The legislator states in Article 61 para. 3 of the Ordinance of 4 May 2022 on In Vitro Diagnostic Medical Devices (IvDO; SR 812.219) that, in principle, the dispensing of such medical devices to the general public is prohibited in Switzerland. Ordinance 3 on Measures to Combat the Coronavirus (Article 24 para. 4bis) specifies an exemption to this prohibition, stating that COVID-19 rapid self-tests listed by the FOPH may be dispensed to the public.