Background
In aesthetic medicine, but also in other medical disciplines, treatments based on the transplantation of the body's own fat and stromal vascular fraction (SVF) derived from this fat are increasingly being offered. To this end, subcutaneous adipose tissue is taken from a patient, prepared in various ways and then transferred to another site on the same patient (autologous) with various objectives, for example adding volume, skin regeneration or the treatment of arthritis. The transplantation of adipose tissue and SVF is regulated by the Transplantation Act (TpA, SR 810.21), the Therapeutic Products Act (TPA, SR 812.21) and the Human Research Act (SR 810.30).
Adipose tissue and derived SVF, which contains adipose stem cells, preadipocytes, fibroblasts, vascular endothelial cells and various immune cells and growth factors, can be classified legally in two groups, each with differing legal requirements.
This publication is designed to explain the basis for the legal classification and corresponding legal requirements pertaining to autologous adipose tissue and derived SVF for autologous transplantation.
Preparation and use of adipose tissue vs. stromal vascular fraction
Prior to grafting, autologous adipose tissue harvested using liposuction is processed in different ways, depending on the target application. The types of preparation can be summarised as follows:
1. Adipose tissue is processed mechanically (e.g. by sedimentation, filtration, centrifugation) to remove blood, serum, destroyed fat cells and unwanted fluids, and then transplanted as prepared adipose tissue, primarily in order to add volume.
2. SVF is isolated by separating the fat cells from the adipose tissue. SVF is increasingly being transplanted in aesthetic medicine or used therapeutically, for example for orthopaedic indications.
The SVF is isolated from aspirated adipose tissue either by means of an enzymatic or mechanical method:
- In the enzymatic method, the aspirated adipose tissue is cleaved by a protease. Next, the oil fraction (consisting of fat cells) is separated from the aqueous fraction via centrifugation to produce the SVF.
- In the mechanical method, the aspirated adipose tissue is liquefied via shear forces through repeated shuffling of the tissue back and forth between syringes to produce an emulsion. Next, the oil fraction is separated from the aqueous fraction via centrifugation to produce the SVF. In practice, specially developed medical devices are often used for the mechanical isolation of SVF.
In contrast with autologous fat grafting, autologous SVF grafting does not primarily involve the transplantation of fat cells, but rather adipose stem cells, preadipocytes, fibroblasts, vascular endothelial cells and various immune cells and growth factors.
Differing legal provisions apply depending on the type of preparation and target application of the prepared adipose tissue or SVF.
Legal classification of adipose tissue and SVF for autologous transplantation
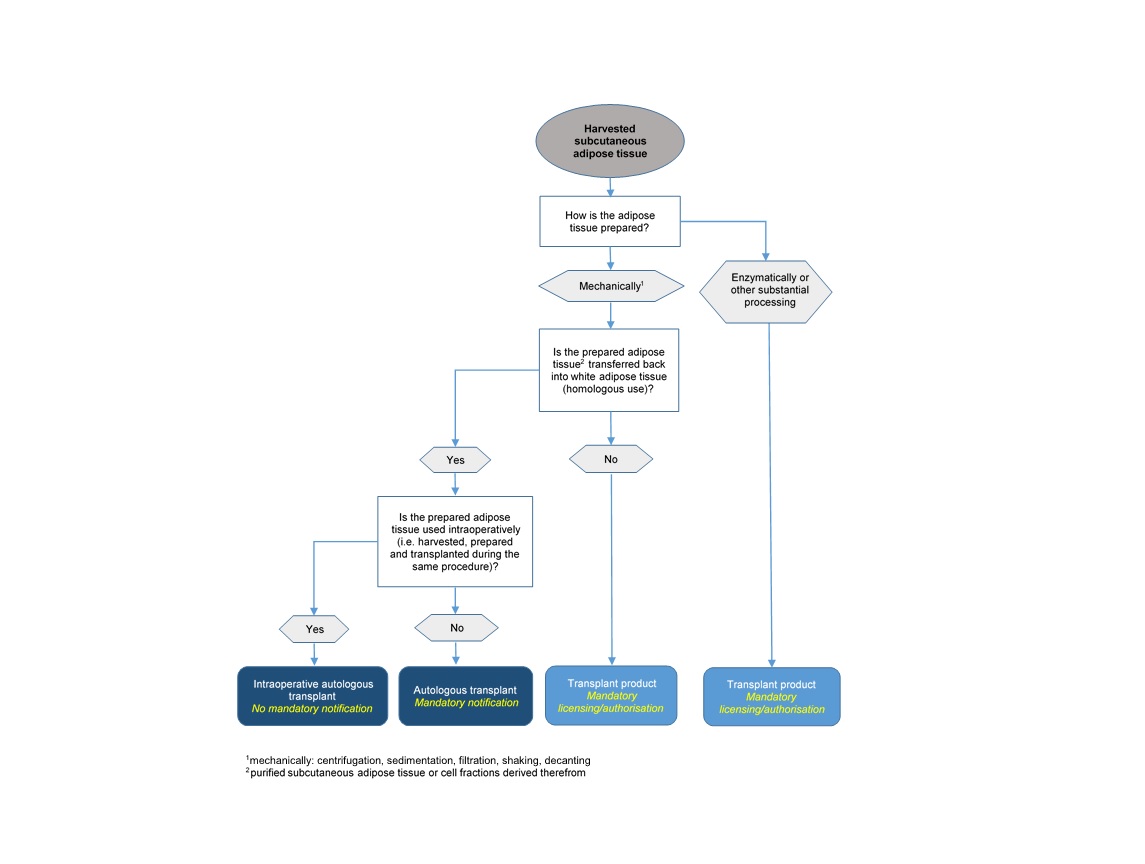
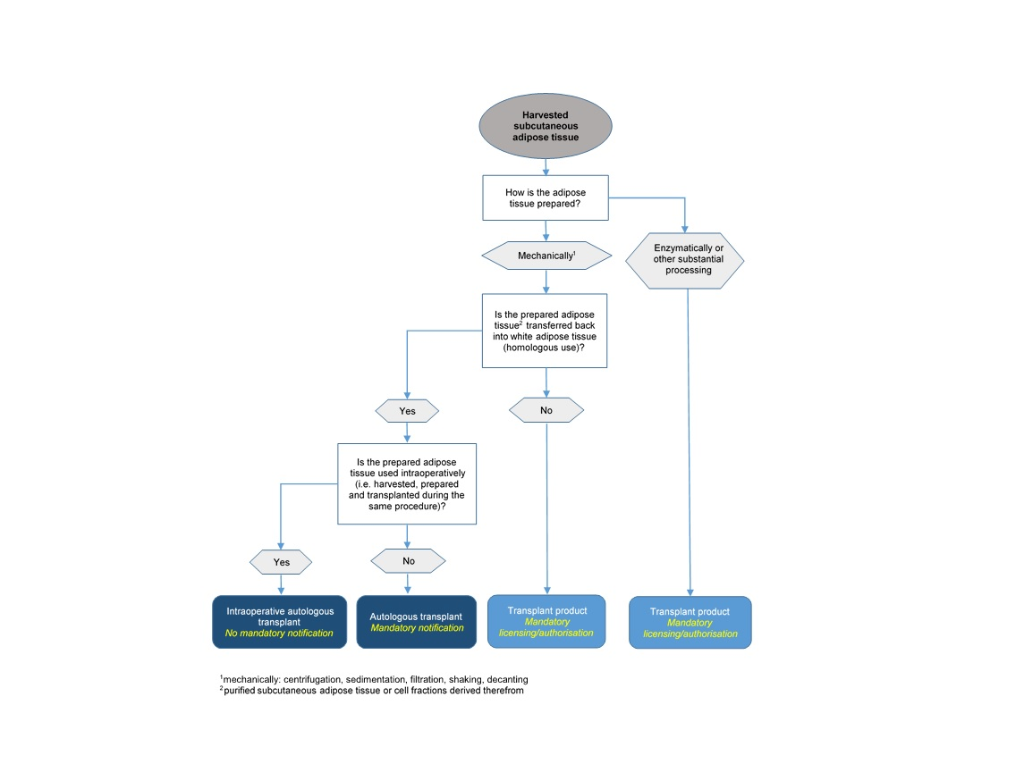
From the legal standpoint, adipose tissue and SVF for autologous transplantation are divided into two groups (Art. 2, Transplantation Ordinance, TpO, SR 810.211), namely:
1. "Autologous transplants" (cells and tissues for autologous transplantation)
Transplants are considered to be tissues and cells that
a. are not processed substantially during preparation, i.e. their physiological properties and functions remain unchanged after the processing steps (minimal manipulation), and
b. perform the same function at the recipient site as at the harvesting site (homologous use).
Autologous transplants are subject to the Transplantation Act and its implementing provisions. Anyone who prepares, stores, forwards, imports or exports autologous transplants must notify Swissmedic accordingly in advance (Art. 15d, TpO). Moreover, prepared tissues and cells must be handled according to Art. 13 and 14 TpO.
According to Art. 1, para. 2 b and Art. 2, para. 1 b, the above-mentioned mandatory notification according to Art. 15d TpO, as well as the requirements for handling cells and tissues according to Art. 13 and 14 TpO, apply to autologous transplants only if they are prepared for "later transplantation". Excluded from this mandatory notification therefore are cells and tissues that are harvested, prepared and transplanted in the patient during the same operation (intraoperative use). However, if the cells and tissues are stored before the autologous transplantation or prepared for later transplantation, this no longer counts as intraoperative use, and the above-mentioned provisions for autologous transplants apply.
2. Transplant products:
According to Art. 2 c and d TpO, transplant products are products comprising, or containing, human organs, tissues or cells, where
a. the organs, tissues or cells have either been substantially processed or
b. do not perform the same function at the recipient site as at the harvesting site (functional change or non-homologous use).
As well as the TpA, transplant products are also subject to the TPA and its implementing provisions. Accordingly, all companies and institutions that manufacture or deal with transplant products must obtain corresponding establishment licences from Swissmedic (Art. 5 and 18, TPA).
For their use in patients, authorisation is also required from Swissmedic for the transplant products themselves (Art. 9, TPA) or their standardisable manufacturing process (Art. 32 of the Therapeutic Products Ordinance, TPO), or else they may be used only in connection with a controlled clinical trial approved by Swissmedic (Art. 54, TPA).
Explanation of the terms "substantial processing" and "functional change"
As described above, a crucial factor in the legal classification of autologous adipose tissue and SVF derived from such tissue is whether the tissues and cells undergo substantial processing and/or a functional change:
1. Substantial processing:
According to Art. 2 d TpO, substantial processing is deemed to be the propagation of cells by cell culturing, the genetic modification of cells and the differentiation or activation of cells.
This definition of substantial processing in TpO also covers the enzymatic manufacturing step for the classification of SVF as a transplant product, since the enzymatic preparation can influence the cell surface, and thus the differentiation and activation of the cells. Consequently, enzymatically prepared adipose tissue/SVF is classified as a transplant product. Mechanical methods, e.g. filtration, centrifugation or decanting, are basically deemed to be non-substantial processing, unless they alter the physiological properties or the function of the tissue or cells.
2. Functional change:
A functional change exists if harvested cells or tissues are not used to perform their original basic function in another part of the human body in the same histological environment.
As subcutaneous adipose tissue is classified as white adipose tissue, only with transplantation back into white adipose tissue can an identical histological environment be assumed. This means that if subcutaneous adipose tissue or cell fractions derived therefrom are not injected back into white adipose tissue for the purpose of adding volume/reconstruction but into a different target tissue (e.g. other loose connective tissue, muscle tissue or dermal tissue) or for another purpose, this is a functional change. In this case, it can no longer be assumed that the tissue/cells will perform their original basic function.
On this basis, the following flowchart is designed to facilitate the legal classification of autologous adipose tissue and derived cell fractions and establish the associated notification, authorisation or approval obligations: