Swissmedic, the Swiss Agency for Therapeutic Products, is fit for the future! And that applies across the board. We will show how we are using innovative approaches to prepare for the future in therapeutic products regulation, what steps we have already taken in digitalisation and what steps still lie ahead. But that’s not all. We have extended our previous familiar "Regulatory News" event and will look beyond the known regulatory issues by offering information all about the life cycle of medicinal products and medical devices.
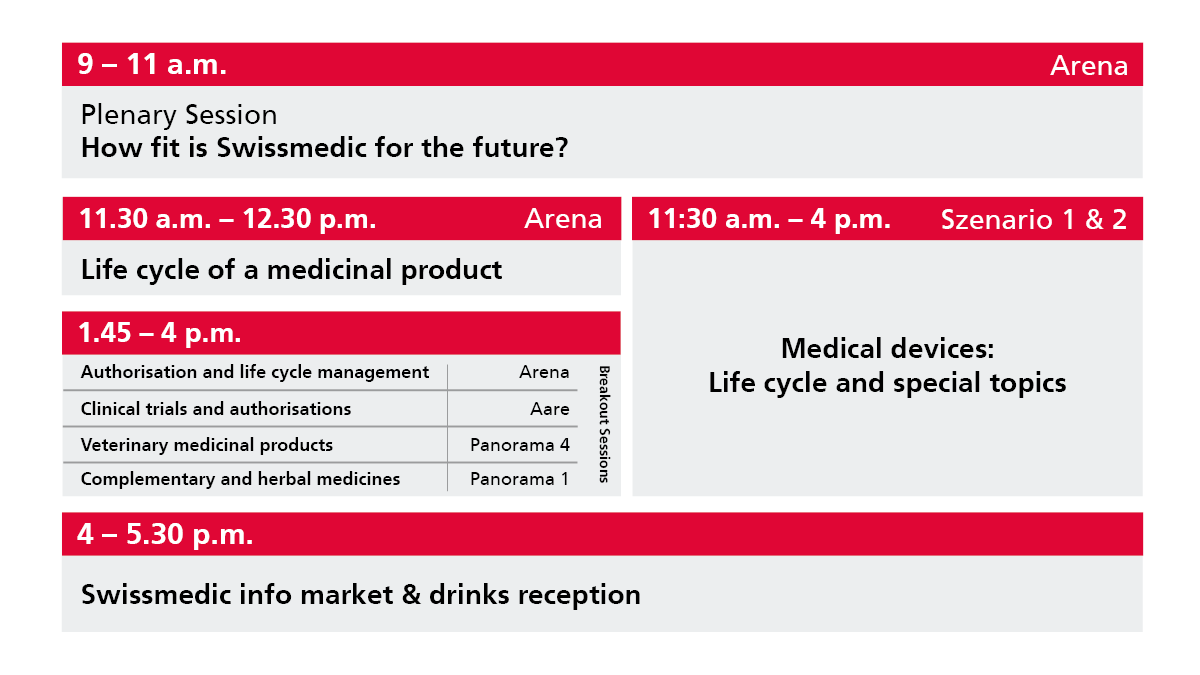
Presentations
Plenary Session: Swissmedic – fit for the future
Medicinal products – Life cycle and special topics
Authorisation & life cycle management
Clinical trials & licensing
Veterinary medicinal products
Complementary and herbal medicines
Medical devices – Life cycle and special topics
Programme outline: Swissmedic Regulatory & Beyond