As at 24 February 2021, Swissmedic, in collaboration with the Ticino reference centre and other regional pharmacovigilance centres in particular, has evaluated 364 reports of suspected adverse drug reactions (ADR) to COVID-19 vaccinations in Switzerland. The reports corroborate the side effects profile identified during the authorisation studies and described in the medicinal product information, and do not as yet point to any new safety issues.
364 reports of suspected adverse reactions to COVID-19 vaccines in Switzerland evaluated
26.02.2021
As at 24 February 2021, Swissmedic had evaluated a total of 364 reports of suspected adverse drug reactions (ADR) in connection with COVID-19 vaccinations in Switzerland. 199 reports involve Pfizer/BioNTech’s Comirnaty®, while 154 are associated with Moderna’s COVID-19 vaccine. In two cases, the vaccine was not specified. The majority of reports were submitted by medical professionals, but 28 (7.7%) came direct from patients or people affected.
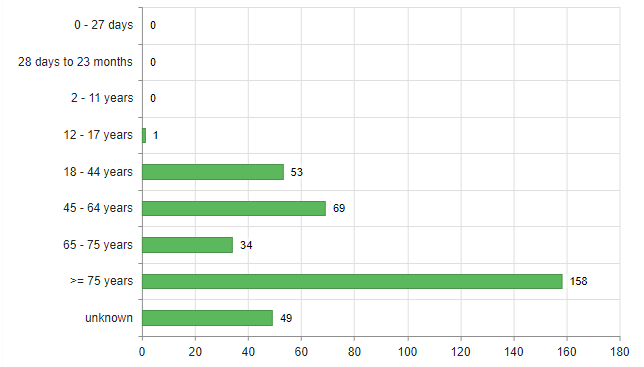
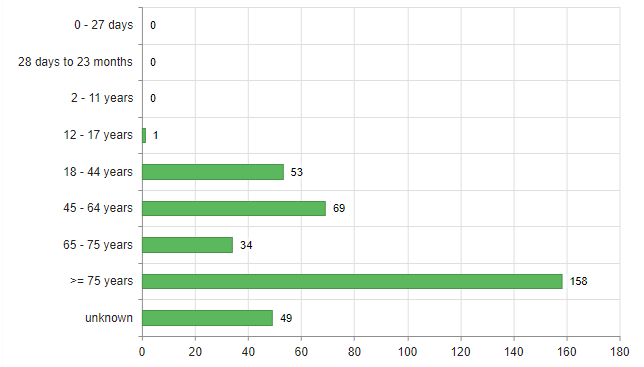
Most reports concerned women (67%). Men were affected in 28.3% of cases, and there were a few cases (4.7%) where no gender was specified. Figure 1 shows the age distribution of the people affected. Of the 158 people aged 75 or over, 81 were between 80 and 89, while 16 were 90 or older.
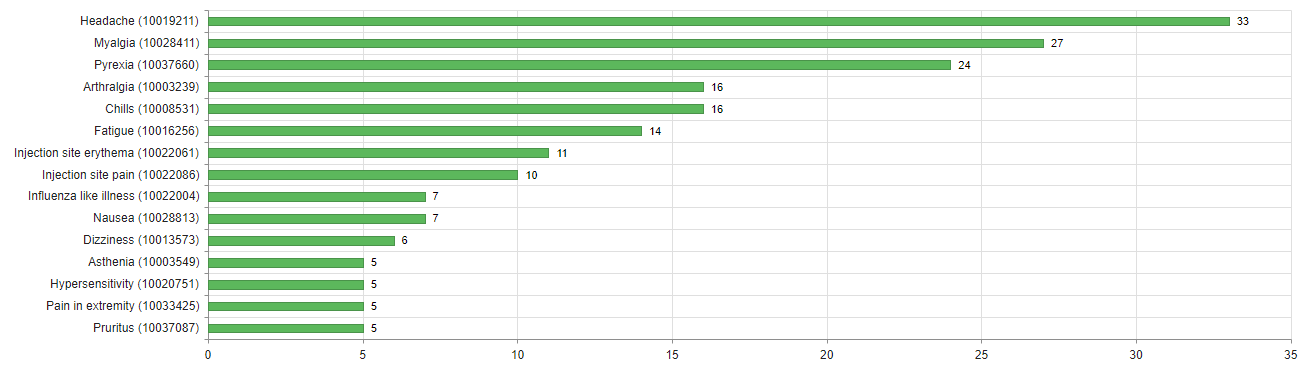
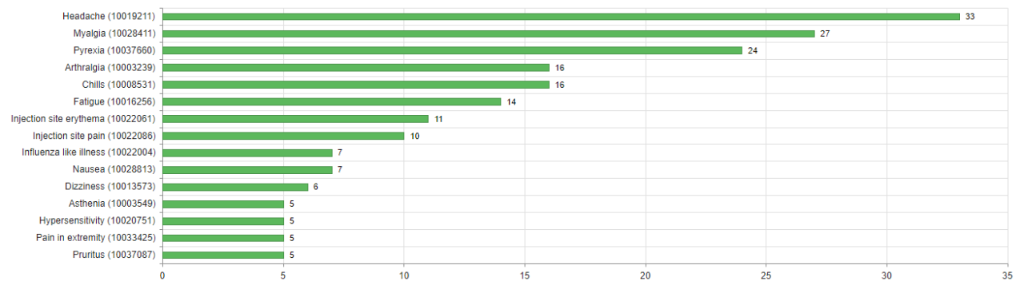
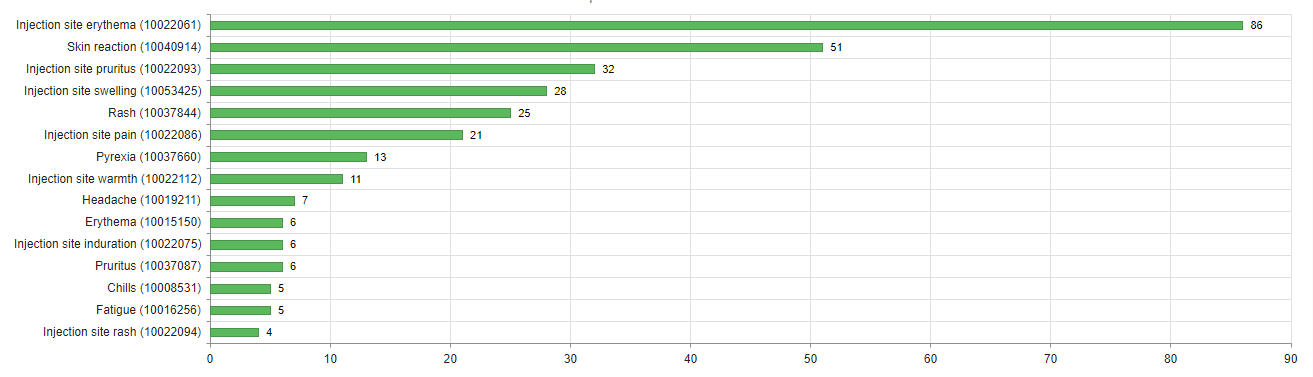
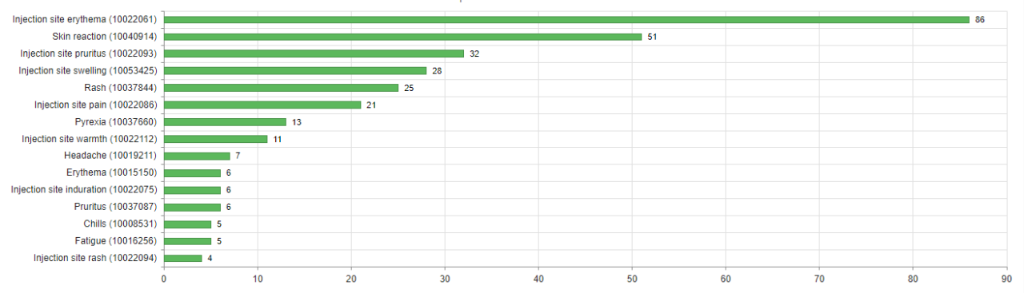
About three quarters of cases (74%) were not serious. The most common reactions in these cases were injection site reactions (redness, itching, pain and swelling), headaches, fever and shivering. Particularly noteworthy were the reports of delayed skin reactions around the injection site or on the skin of the arm in which the vaccine had been administered, which appeared to occur more frequently with Moderna’s COVID-19 vaccine (see figures 2 and 3). Swissmedic has already provided information on these reactions.
There were 95 serious cases (26%). The people affected were either treated in hospital or their reactions were classified as medically significant for other reasons but were not in themselves dangerous in most cases. The most commonly reported reactions in these cases were shingles (n=8), fever (n=8), headache (n=7), COVID-19 (n=9) and allergic reactions, including four cases of anaphylactic reactions (see also Swissmedic's communication of 29 January 2021).
Since a single report generally involves more than one reaction, reactions such as fever or headache are reported in cases that are serious overall as well as in non-serious cases.
In 16 serious cases, the people concerned died at differing intervals after receiving the vaccine. Their average age was 86, and the majority of them had serious pre-existing conditions. These cases were analysed against the available information with particular care. As far as is known at the moment, death was caused by conditions such as infections, cardiovascular events, or diseases of the lungs and airways. Despite a chronological correlation, there is no concrete evidence to suggest that the vaccination was the cause of death. The drug regulatory authorities of other countries and the WHO have also drawn similar conclusions when evaluating deaths with a temporal link to COVID-19 vaccines.
The reports of adverse reactions received to date and subjected to careful analysis do not change the positive benefit-risk profile of the COVID-19 vaccines. The known side effects of COVID-19 vaccines are listed in the continually updated medicinal product information published on www.swissmedicinfo.ch.
Information on reporting ADR associated with COVID-19 vaccines
Please note: Known, non-serious reactions are not subject to the reporting obligation prescribed by Art. 59 of the Therapeutic Products Act. The known, non-serious and very common reactions to COVID-19 vaccines include transitory pain and swelling at the injection site, fatigue, shivering, fever, headache and muscle and joint pain. These passing local and general reactions are normally a sign of the body dealing with the vaccine.
However, serious or as yet unknown adverse reactions must be reported. At this relatively early stage of the vaccination campaign, doctors should generally report all ADR that they feel are medically relevant. For this purpose they should use the ElViS electronic reporting tool, which they also can log into using their HIN ID.