Asian medicinal products are considered to be complementary medicines and, in accordance with Chapter 7 KPTPO[1], comprise medicinal products with and without indication from the three definitively established schools of Asian therapy.
Other valid documents can be found at:
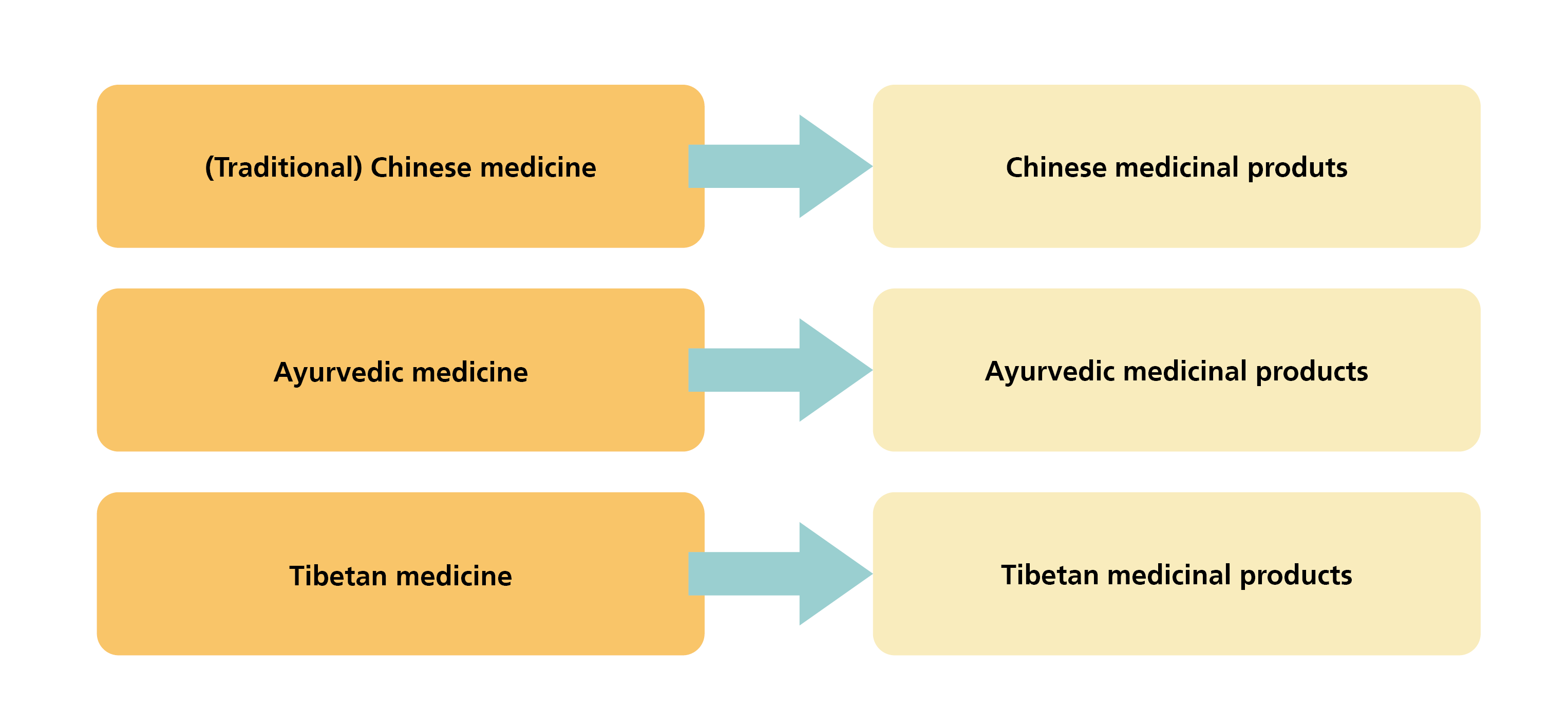
The substances contained in Asian medicines, the preparations manufactured from them and the compositions, dosage forms and indications are derived from the corresponding tradition and reflect the approach of the Asian therapy in question.
The indications for medicinal products with indication are determined and approved in accordance with the approach of the therapy in question, while medicinal products without indication are used for individual treatment by specialists in the respective therapy.
Different procedures may be used to authorise Asian medicinal products as defined in Chapter 5 KPTPO depending on the conditions and requirements that need to be fulfilled:
------------
[1] Ordinance of the Swiss Agency for Therapeutic Products on the Simplified Authorisation of Complementary and Phytotherapeutic Products (SR 812.212.24)
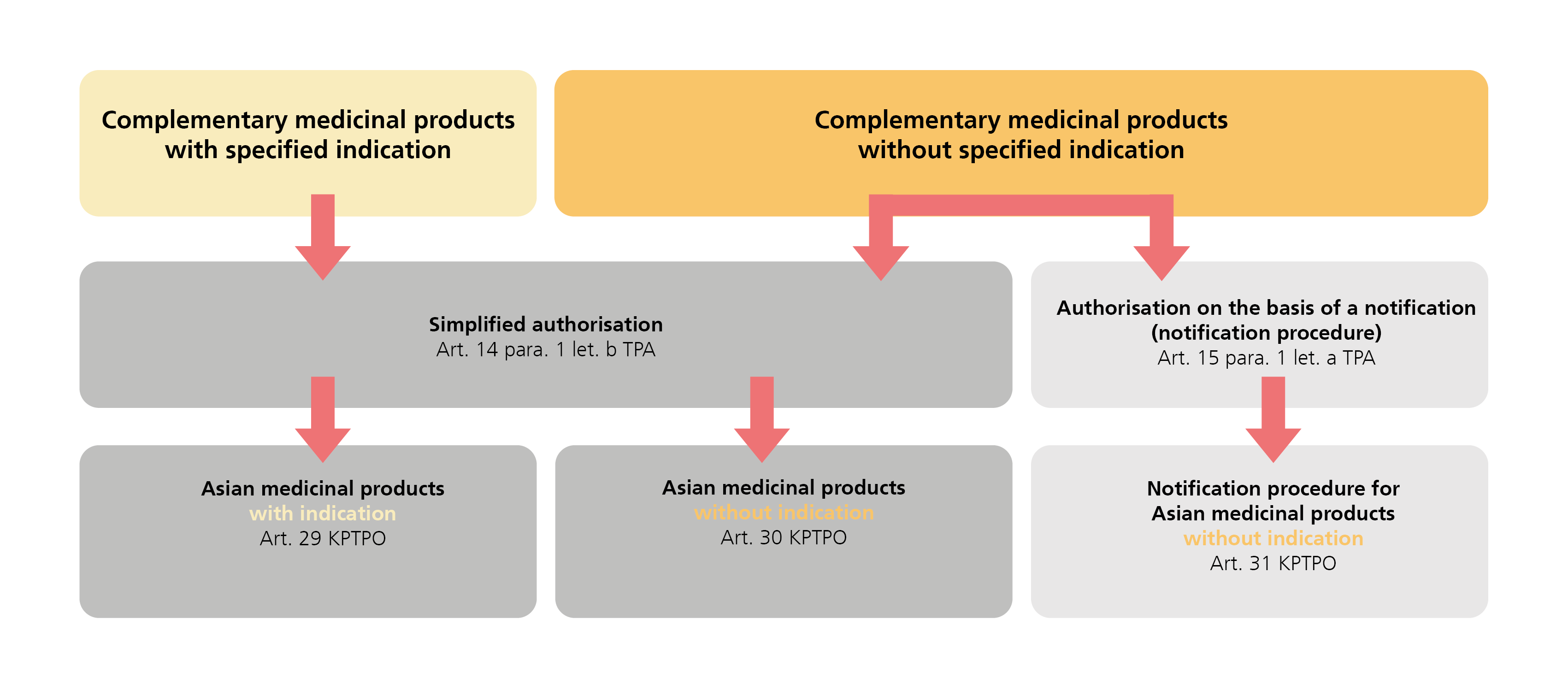
The procedures differ in terms of the scope of the evidence that must be submitted with an application for authorisation.
Further information can be found in the Guidance document Authorisation of Asian medicinal product HMV4.

