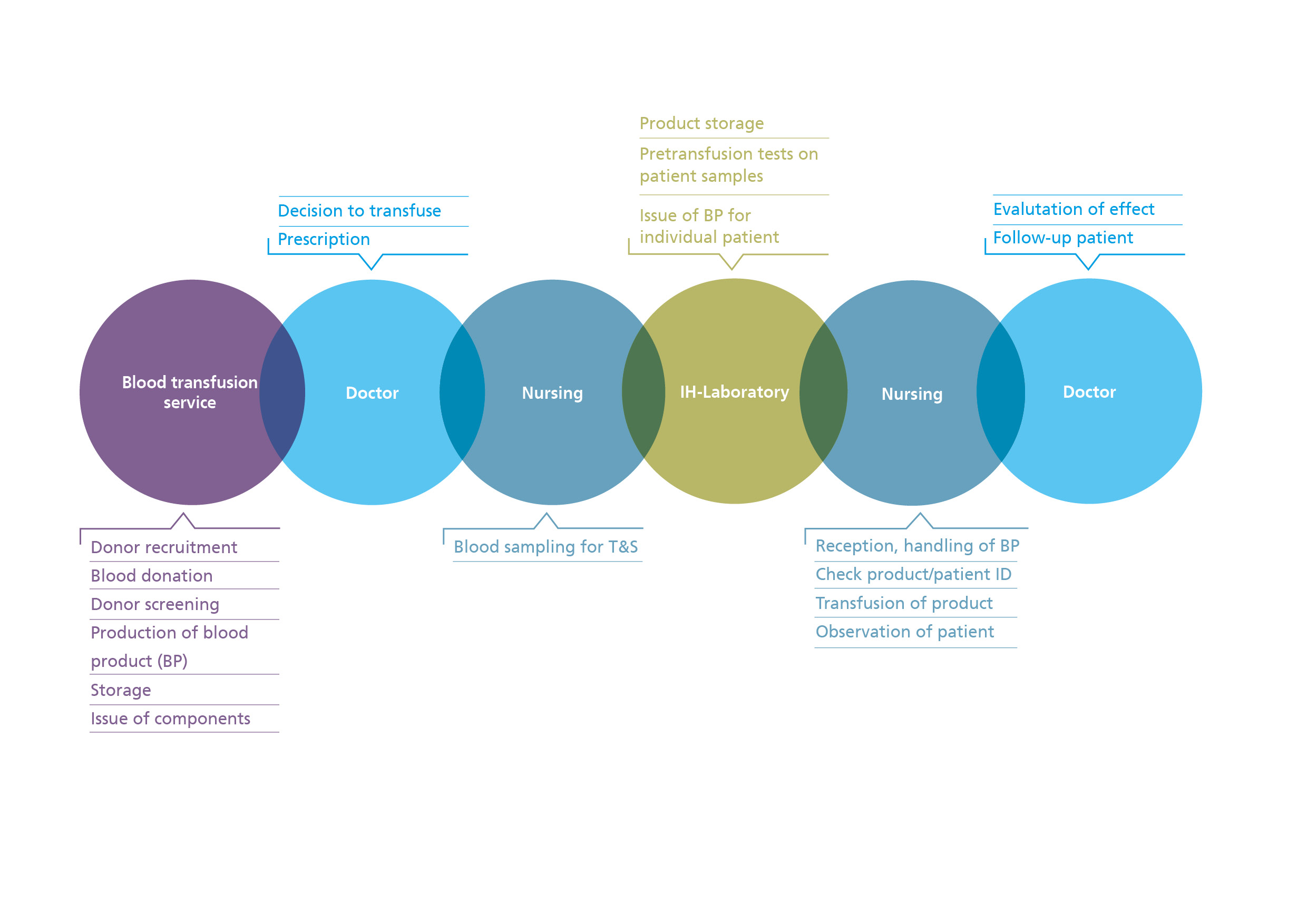
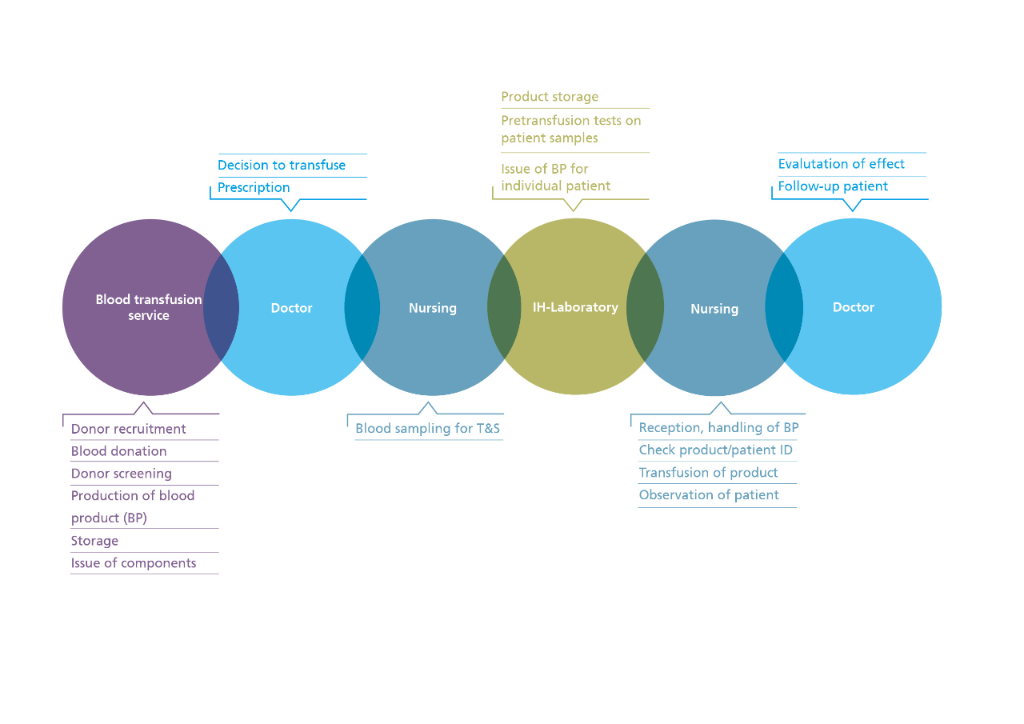
Targeted haemotherapy means that patients are given the correct, safe and effective blood product on time. This requires all the individuals concerned to take their share of responsibility, starting with the blood donor, the blood transfusion services as producers of labile blood products, the transfusion laboratories, the treating doctors and the authorities.
Despite all the safety precautions, adverse effects must be expected when blood and labile blood products are manufactured and used. The systematic compilation and evaluation of data from all links in the transfusion chain allows transfusion risks to be identified and safety in the chain to be increased. These data include side effects experienced by donors, protective measures taken when an infection is detected, reports of quality defects, transfusion reactions and serious incidents associated with the handling of labile blood products, such as transfusion errors or errors that are discovered and corrected in time (referred to below as "near misses").