The following applies in general to GCP and GVP inspections:
- Inspectors are subject to a professional secrecy obligation in accordance with Art. 61 of the Therapeutic Products Act (TPA; SR 812.21).
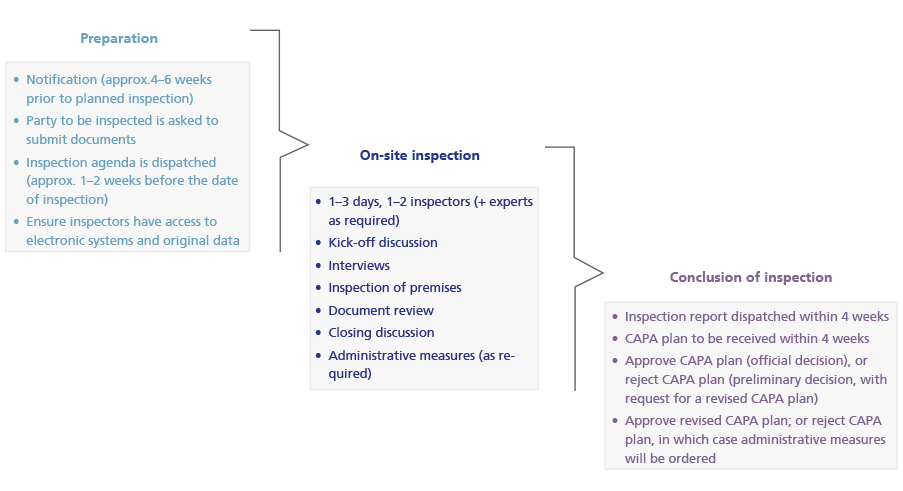
- The addressee for routine inspections is the sponsor or sponsor’s representative in Switzerland (GCP) or the authorisation holder (GVP). Associated correspondence (notification, preliminary and official decision) will take place in the appropriate Swiss national language for the region in which the sponsor, sponsor’s representative or authorisation holder has its registered office. The inspection report will be written in English.
- On receiving the inspection report, the addressee is required to submit a corrective and preventive action plan (CAPA plan) within four weeks.
- CAPA plans of insufficient quality will be rejected. The duration – and thus the costs of the inspection procedure – are heavily dependent on the quality of the CAPA plan submitted by the addressee.
- The addressee will be invoiced for the costs of the inspection, which will be calculated in accordance with Art. 4 of the Ordinance of the Swiss Agency for Therapeutic Products on its fees (Of-Swissmedic, CC 812.214.5).
- For both GCP and GVP inspections, a distinction is made between routine and for-cause inspections.
- The purpose of for-cause inspections is to investigate specific circumstances in the event of suspected gross breaches of applicable legislation or official decisions or a suspected risk to health. For-cause inspections can be carried out without prior notification.
- Routine inspections are also intended to review specific circumstances, but focus on identified risks. A diagram showing the procedure for routine inspections is given below.