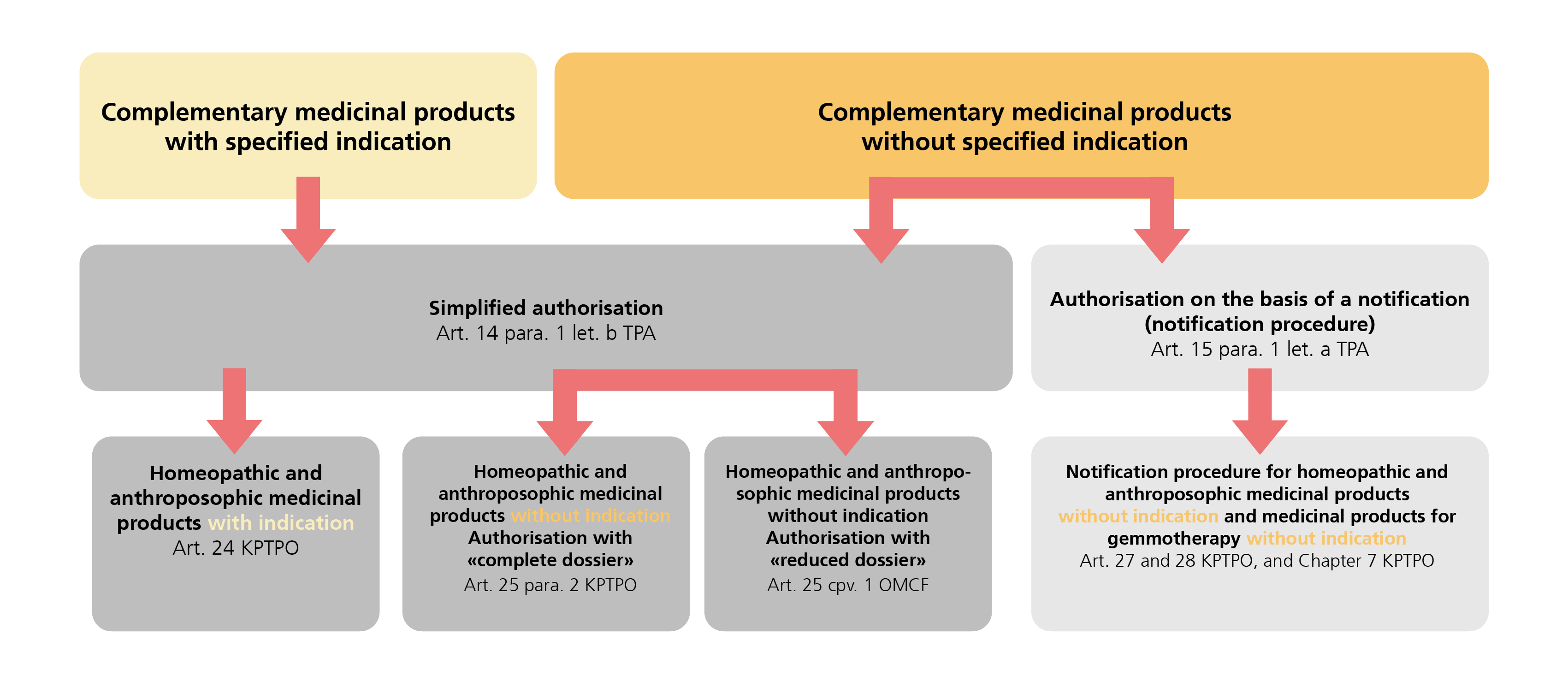
Homeopathic and anthroposophic medicinal products are classified as complementary medicines and, as defined in Chapter 4 of the KPTPO (SR 812.212.24), comprise medicinal products with and without indication and the associated active substances that are manufactured using homeopathic, anthroposophic and spagyric methods and intended for use in accordance with the principles of homeopathic, anthroposophic or spagyric medicine or the biochemical treatment concept according to Dr Schüssler (Schüssler therapy/Schüssler salts).
The indications for medicinal products with indication are determined and approved in accordance with the approach of the therapy in question, while medicinal products without indication are used for individual treatment by specialists in the respective therapy.
Different procedures may be used to authorise homeopathic and anthroposophic medicinal products as defined in Chapter 4 KPTPO depending on the conditions and requirements that need to be fulfilled: