Swissmedic issues warning about the increasing number of falsified, misleading or unauthorised weight loss products currently on offer that make reference to GLP-1 active substances. Such preparations may contain untested, undeclared or harmful substances, and may also be of insufficient quality or contain the incorrect dosage. The use of these products carries significant health risks. Furthermore, “slimming preparations” are now also being sold as nutritional supplements in addition to illegal weight-loss injections. These use misleading names, make fraudulent use of the Swissmedic logo or carry invented quality marks with the aim of deliberately deceiving consumers.
Use of illegally marketed “slimming aids” may carry significant health risks
26.08.2025
GLP-1 receptor agonists currently have a strong social media presence, often accompanied by unrealistic promises of weight loss. Criminal counterfeiters and questionable suppliers are deliberately exploiting this trend by promoting these products – sometimes very aggressively – on social media, websites or via direct advertising in particular. Swissmedic has noticed several concerning trends as regards unauthorised or in some cases falsified products that make reference to GLP-1.
- Misleading presentation
The number of products with labelling such as “GLP-1” is constantly growing. They are marketed as nutritional supplements or drops and may make fraudulent use of the logos of regulatory authorities such as Swissmedic and carry invented quality marks with the aim of deliberately deceiving consumers. - Undeclared active substances
Products that are frequently marketed as “natural” actually contain pharmacologically active substances – without any declaration or dosage information. - Falsifications and unauthorised imitation products
Criminals falsify the labels of commercially available pens or supply active substances that are still being researched as illegally manufactured preparations – in some cases with potentially life-threatening consequences.
Swissmedic urges consumers not to buy and use such products. Taking or using them carries a significant health risk.
Misleading products with fraudulent authorisation logos
Capsules, drops or nutritional supplements are increasingly being advertised on social media with wording such as “GLP-1” or “GLP-1 formula”. The packaging of some of these products carries the logos of state therapeutic products agencies, such as Swissmedic (Switzerland), the FDA (USA) or BfArM (Germany), as well as other “imaginary certifications”. Such labelling is misleading and deceptive, since none of these products have been tested or authorised by any of the authorities named above. There is no indication of medical efficacy or, in particular, weight loss. Consumers are being deliberately deceived.
Undeclared active substances
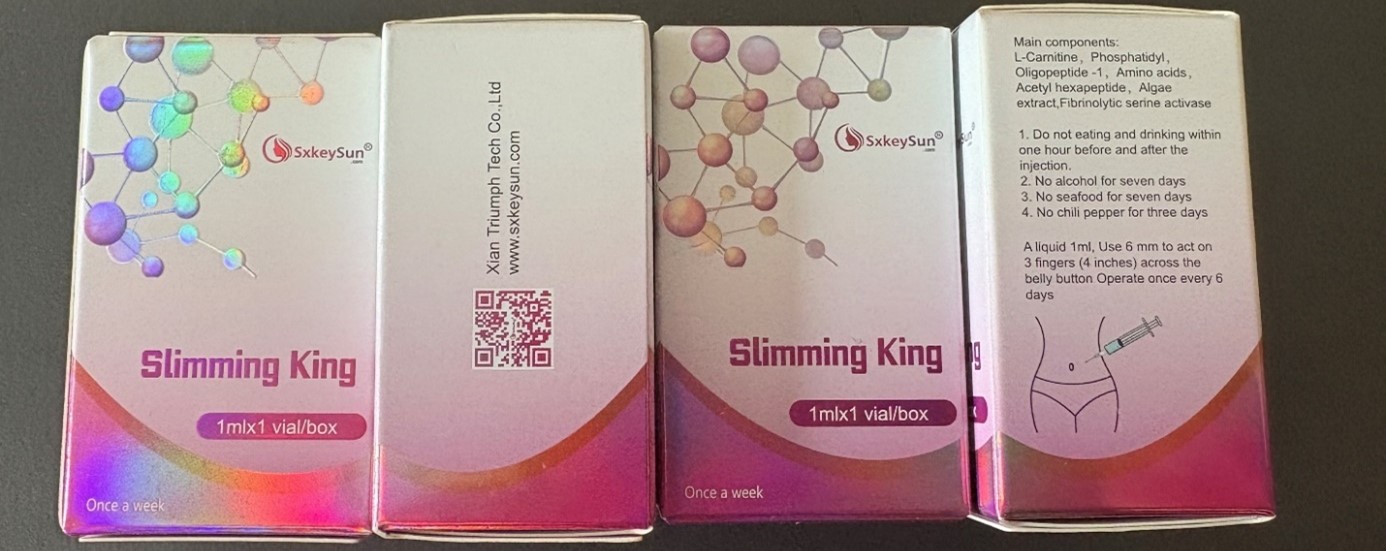
Swissmedic issues regular warnings about weight loss products that are allegedly “entirely natural” but which in reality contain undeclared, pharmacologically active substances. Such falsified products are illegally offered for sale by online sources. One current case involves a confiscated injectable product from Hong Kong branded as “Slimming King”. According to the declaration, the product only contains natural ingredients. Swissmedic’s laboratory found therapeutic quantities of the prescription-only active substance semaglutide in the preparation. Semaglutide can only be taken under medical supervision.
Products with undeclared active substances are particularly risky. Anyone who uses such preparations is undertaking drug treatment without medical supervision. Medically unsupervised use can have serious health complications.
Furthermore, Swissmedic reminds consumers that products for injection may only be marketed as therapeutic products. Consumers should be particularly wary of injectable preparations on sale in cosmetic studios, beauty salons or similar outlets because in many cases the products in question are illegal.
Falsified and unauthorised imitation products containing GLP-1 active substances
When GLP-1 products first began to attract public interest, there were repeated cases of products being falsified by label swapping. This involved attaching labels from medicinal products containing GLP-1 to commercial insulin pens and selling them under the guise of slimming aids. The result of healthy people injecting themselves with insulin can be acute, life-threatening hypoglycaemia. Reports of several medical emergencies reached Switzerland in this context. The external similarity of the pens can lead to confusion about the actual substance contained in them and carries a serious risk of mix-ups.
Illegal suppliers who used to specialise in doping and anti-ageing preparations have now also started to sell GLP-1 analogues such as semaglutide through online channels – generally on its own as powder or a solution, and often promoted by influencers. The active substances frequently come from Asia and are not subject to any pharmaceutical control. Even though the product presentation may look professional, there is absolutely no guarantee of quality, efficacy and safety. Laboratory testing reveals chemical and microbiological impurities that may endanger health. The Swissmedic laboratory found an impurity in one sample that could trigger hypersensitivity reactions in sensitive individuals.
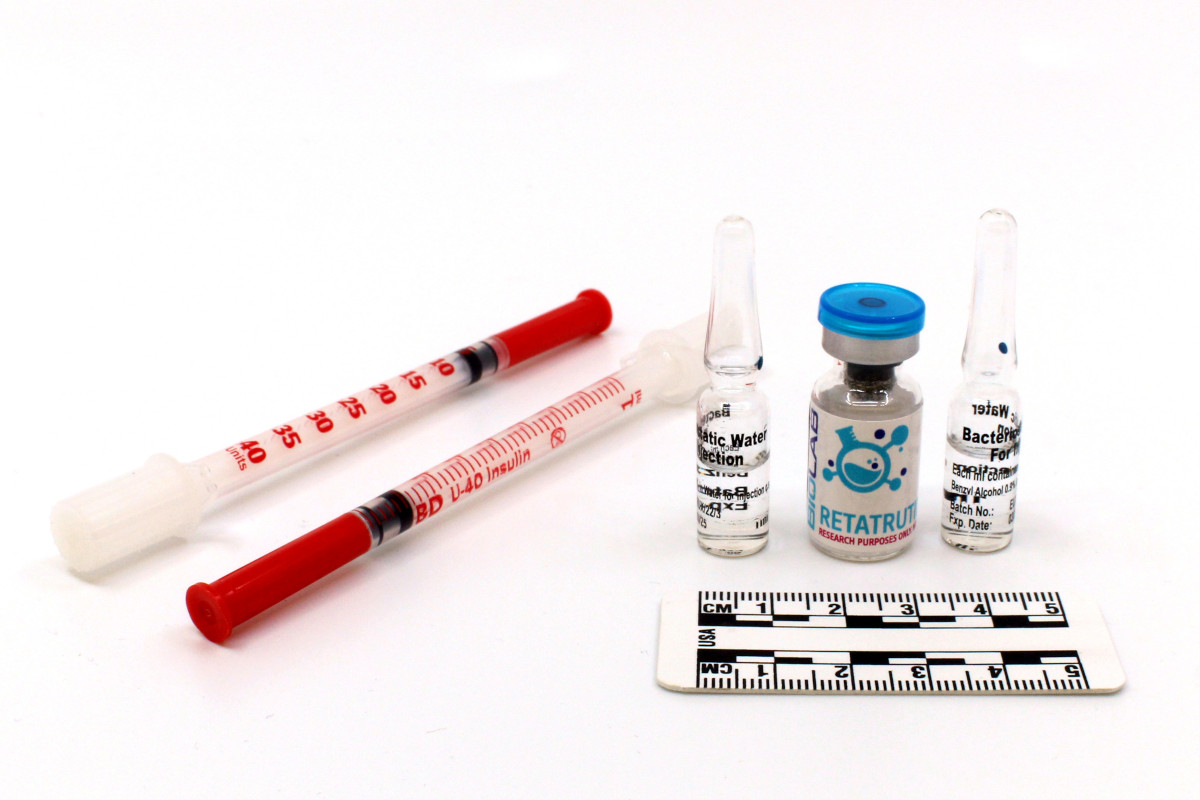
Some suppliers are also selling new peptide active substances, the efficacy of which is not yet clinically proven. One example is the “retatrutide injection kit”: The substance contained in the kit is still in clinical development and is not authorised internationally. Nevertheless, it is already available on the black market. The safety, efficacy and quality of such preparations cannot be verified owing to their dubious origin and lack of regulatory control.
Active substances for blood sugar regulation and weight loss (GLP-1 receptor agonists) are only available on prescription and must be used under medical supervision. Using them at the recommendation of influencers or ordering them from uncontrolled, questionable suppliers represents a specific danger to health.
Unauthorised or falsified medicinal products carry considerable risks – particularly if they contain undeclared substances, impurities or incorrect doses. Swissmedic urges consumers not to purchase medicinal products or medical devices from unknown sources on the Internet or social media.
See also
Warning about supposedly herbal products
Illegal medicines and the internet
[ Video 02:00 ]
Market surveillance: "Thumbs up or thumbs down"
Portrait of the unit for Market Monitoring of Illegal Medicinal Products
Supplementary information
Swissmedic: National single point of contact for combating falsified therapeutic products
Swissmedic checks reports of suspected illegal products, introduces corrective measures, initiates administrative and criminal proceedings or forwards the reports to the competent national or international agencies.
Swissmedic: Vital hub in the joint campaign to combat therapeutic products crime
Address for enquiries
Swissmedic
Media Unit